Mrs Capa
Active Member
- Joined
- Jul 21, 2012
- Messages
- 35
- Reaction score
- 0
This has to be the worst soap I have made ever... I'm going to have to grate it up for laundry soap. For some reason it is lye heavy after using the same oil water and lye content as a previous batch that came out wonderfully nourishing with EOs. The difference? I used Fragrance Oils this time. I am normally an Essential Oils soaper exclusively. I hope it wasn't a gremlin in my weighing scales! :shock: Maybe some one here has had a similar experience. :?:
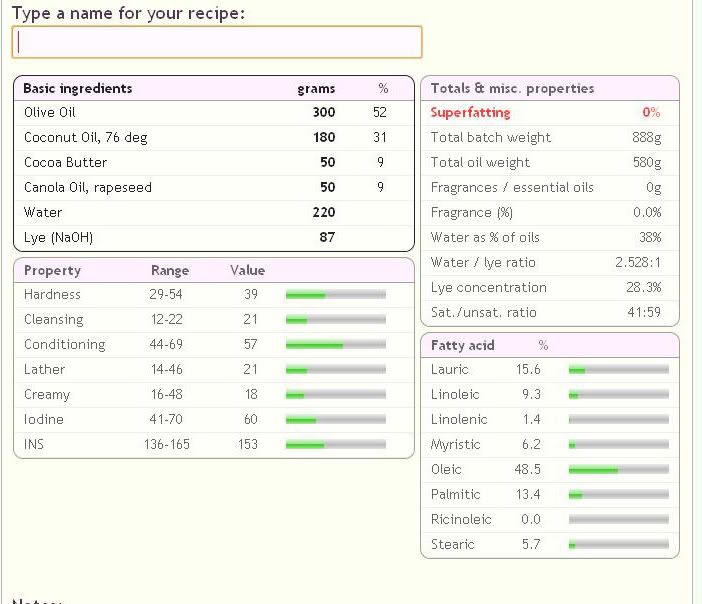
300g Olive
180g Coconut
50g Canola
50g Cocoa Butter
50g Creamed Coconut (63% fat)
220 Water
87 Lye
3% FO + 5ml Nutmeg EO
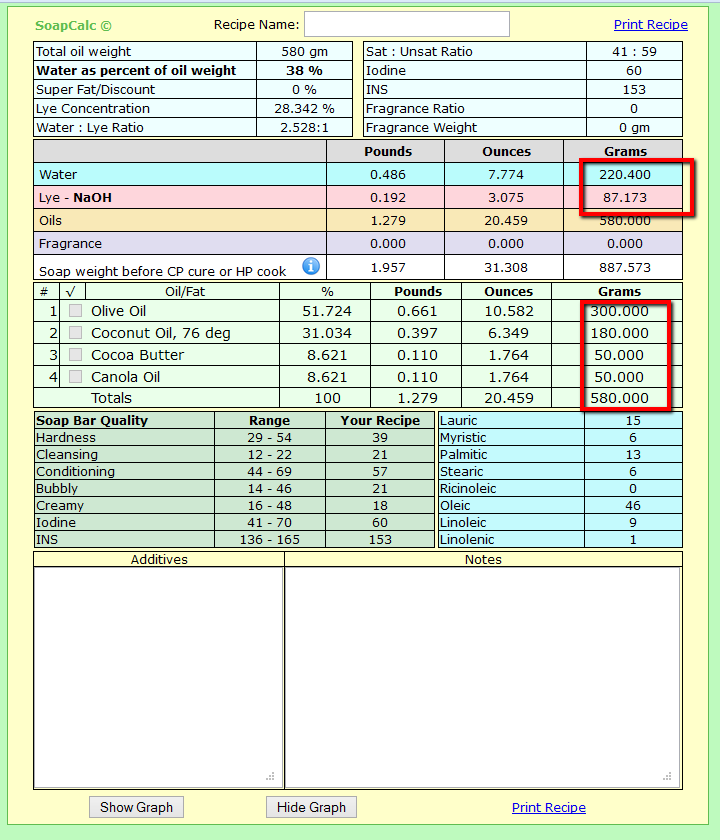
300g Olive
180g Coconut
50g Canola
50g Cocoa Butter
50g Creamed Coconut (63% fat)
220 Water
87 Lye
3% FO + 5ml Nutmeg EO